Etusivu " Navigating the Complexities of High-Stakes Injection Molding
The gap between a validated design and a reliably manufactured plastic component is where ambitious product timelines go to stall.
A validated design confirms that a device works as intended; reliable manufacturing proves that its injection molded parts can be produced at scale with consistency and precision. This Closing this gap demands Design for Manufacturability (DFM) tailored for injection molding, robust process validation, and disciplined design transfer process.
For engineers in MedTech, Liikkuvuusja SmartTech, this isn’t just a manufacturing challenge—it’s a precision engineering and systems integration problem.
Factors like the mechanical behavior of polymer material, tolerance stacking across multipart assemblies, and the strict requirements for documentation and traceability – especially for regulatory compliance —significantly increase complexity. Often, the traditional model of engaging a series of specialized vendors introduces significant risk, from intellectual property fragmentation to communication gaps that compromise part quality.
The strategic advantage lies in treating injection molding not as a separate phase, but as an integrated extension of the product development cycle. The early integration of manufacturing expertise into the development process creates the conditions for reproducible quality, shorter time-to-market, and more reliable regulatory compliance.
A Framework for Mitigating Risk in Precision Components
A robust risk-mitigation framework for precision components begins at design phase, is reinforced through validated manufacturing processes, sustained by stringent production controls, and continuously refined by feedback loops and vigilant governance.
Here are three critical pillars for de-risking complex projects:
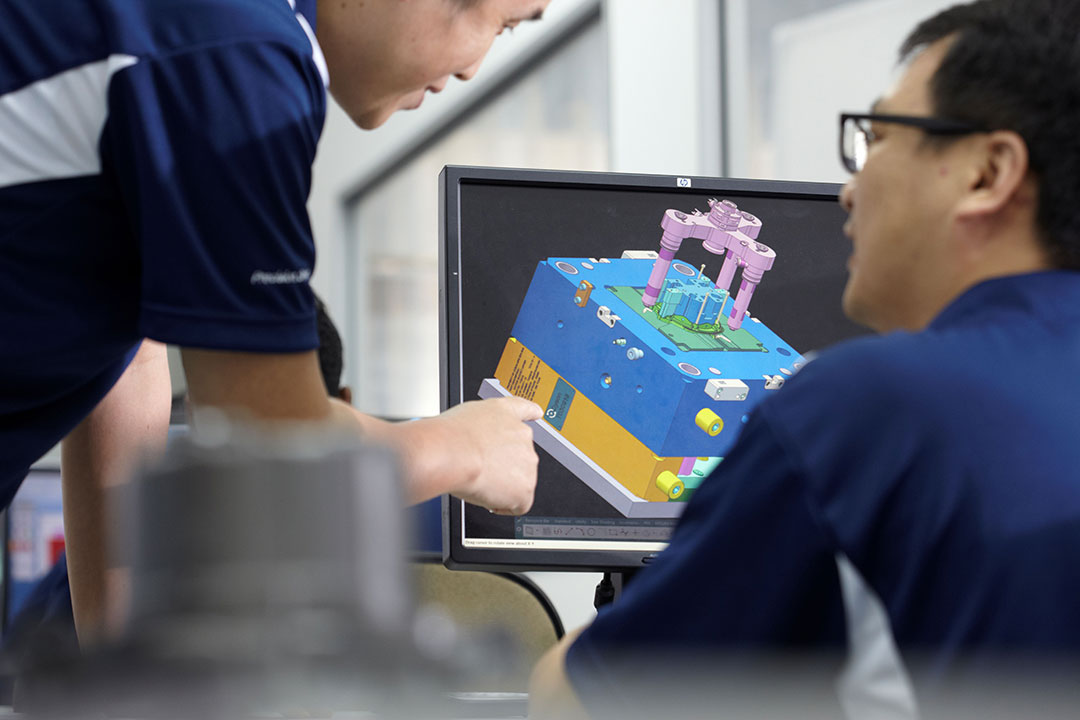
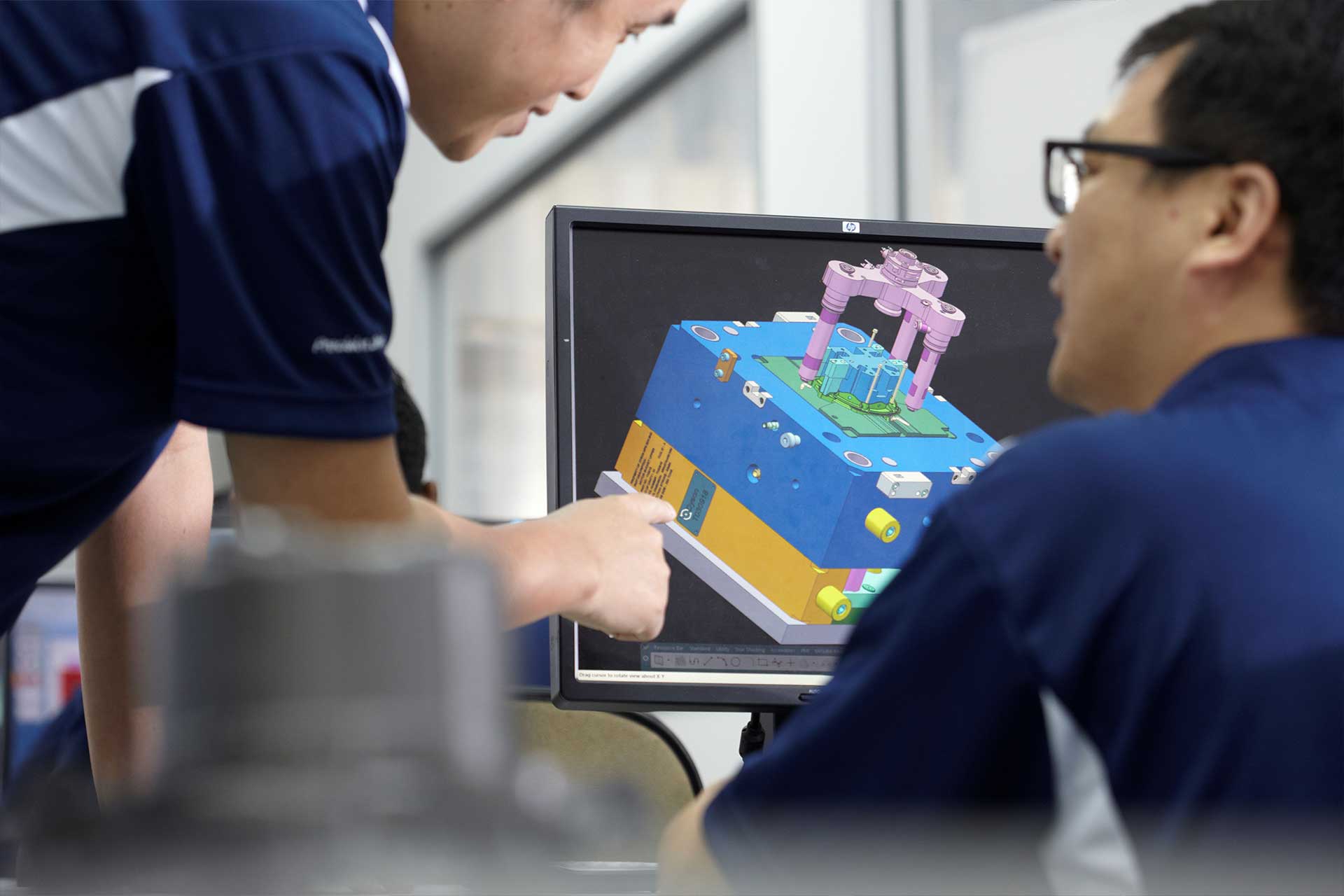
1. Early-Stage Collaboration and Virtual Validation.
Early-stage collaboration aligns design intent with manufacturing capabilities, while virtual DFM (Design for Manufacturability) validation identifies potential risks in the digital realm — long before they can impact cost, quality, or timelines in the physical world.
At Beyonics, we integrate this analysis directly into the DFM feedback we provide partners, selecting the right polymer for the application—balancing factors like chemical resistance, biocompatibility, mechanical properties, and cost. The goal is to design for manufacturability without compromising the design intent.

2. Controlling the Variables Through Vertical Integration.
Inconsistency and variability are the most significant challenges in plastic manufacturing, particularly for precision or regulated industries like medical devices. The path to achieving process stability is built on material discipline, scientific molding, comprehensive process monitoring, rigorous tooling maintenance, and digital quality systems.
This is the core of the Beyonics advantage: we consolidate the entire value chain under one roof—from tool design ja precision molding (including injection molding, insertin muottivalu, overmolding, and 2K molding) to secondary operations like ultrasonic welding, and final quality inspection. This integrated approach eliminates the friction of a fragmented supply chain, ensuring consistently high quality from prototype to full-scale production.
This provides a single point of accountability and a closed-loop data system, ensuring traceability from raw material to shipped unit.

3. Designing for Scalability and Compliance from the Start.
Scalability and compliance are not afterthoughts — they must be integrated into the design from day one. Early alignment between design, manufacturing, and regulatory teams ensures that products not only function in the lab but can also be reliably manufactured, scaled, and approved for global markets.
A prototype is very different from a high-volume production part. The mold design, machine selection, and quality metrics must be engineered for repeatability at scale. For regulated industries, this means designing a manufacturing process that is itself validated and auditable. Our in-house tooling and automated production lines are engineered specifically for this transition, allowing for a seamless shift from pilot runs to mass production without costly re-engineering or qualification delays. Every parameter is documented, and every lot is traceable.
Engineering as a Partnership
The most successful product launches are characterized not only by innovative design but also by the upfront integration of manufacturability, compliance, and customer value. This ensures a seamless transition from prototype to global scale.
This requires a shift in thinking—away from purely transactional relationships and toward genuine technical partnerships.
At Beyonics, we live this partnership model. We apply an integrated framework to transform complex designs into manufacturable, scalable, and compliant realities.
The question isn’t just who will build your part, but how the entire process will be engineered for success.
Do you require precision plastic components? Our engineering team is ready to support you—from initial concept to full-scale production.
Contact our engineering team to discuss your specific requirements.
With decades of experience serving MedTech, Mobility, and SmartTech leaders, our methodology meets the exacting standards of modern manufacturing. Partner with Beyonics to leverage our metal and plastic tooling expertise for your next innovation.